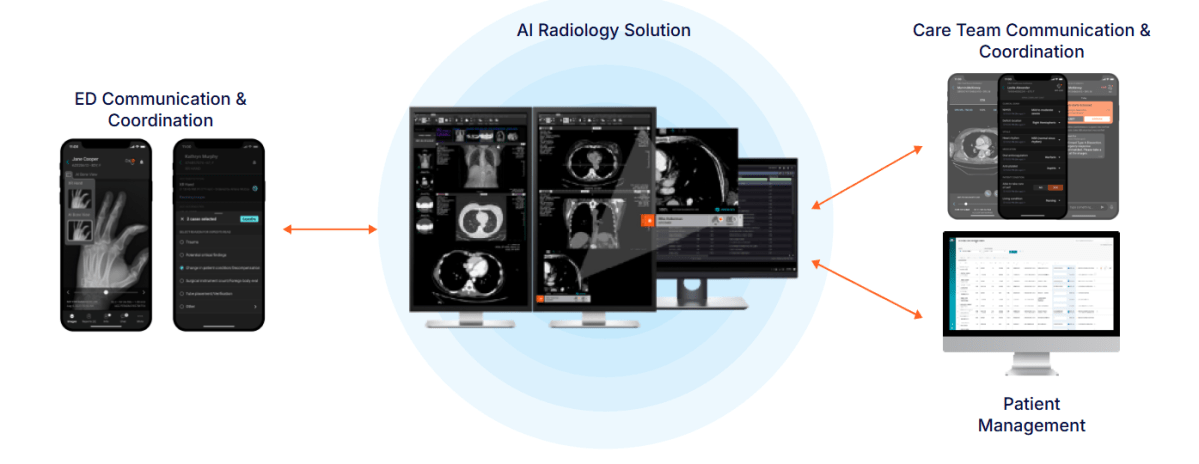
Aidoc has received FDA clearance for a comprehensive AI-powered triage solution designed to help health systems identify critical findings earlier and reduce delays caused by emergency department crowding and imaging backlogs. The solution is powered by CARE, Aidoc’s proprietary AI foundation model, and brings together 11 newly cleared indications with three previously cleared indications into a single, unified workflow.
The platform enables simultaneous triage of multiple acute findings during periods of high clinical demand, creating a safety net that helps surface urgent conditions earlier—both in emergency settings and in ambulatory environments where routine scans may sit in long backlogs. The FDA clearance validates Aidoc’s foundation-model approach, marking what the company says is the first FDA clearance of double-digit acute indications powered by a single AI model. Compared with traditional single-condition tools, the system delivers significantly improved signal quality, reducing false alerts by roughly an order of magnitude.
In an FDA-reviewed pivotal study, the 11 newly cleared indications achieved an average 97% sensitivity and 98% specificity, performance levels that support real-world clinical adoption. The solution is delivered through Aidoc aiOS, the company’s enterprise AI operating system, which handles data normalization, continuous performance monitoring, and governance—allowing health systems to deploy multi-condition AI without reworking existing infrastructure. Aidoc plans to expand CARE across all CT and X-ray workflows over the next 18 months and is developing additional capabilities such as automated draft report generation to support end-to-end clinical AI workflows.
Reference: https://hlth.com/insights/news/aidoc-wins-fda-nod-for-comprehensive-foundation-model-ai-2026-01-26